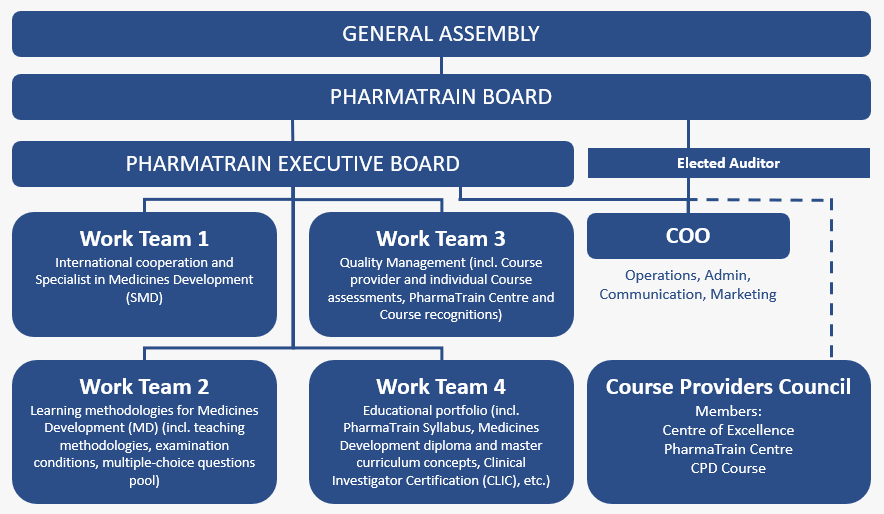
Governance
Board & Executives
PharmaTrain is a network of public and private partners that started its activities as an IMI (Innovative Medicines Initiative) European Project in 2009 and has become a permanent not-for-profit organisation as of July 2014.
The "European Federation of Course Providers in Pharmaceutical Medicine, EFCPM" in Switzerland was formed in 2009. It was originally formed to become the joint consortium partner of course providers in the IMI project "PharmaTrain".
In 2012 EFCPM was renamed to "PharmaTrain" and started its activities as an IMI European Project.
After the completion of the IMI European Project in April 2014, The PharmaTrain Federation was created in Switzerdland to ensure the sustainability of the initiative beyond the Project Frame and receive the last EU Fundings.
Due to insecurity of future EU funding to Switzerland in light of the envisaged application to a new IMI call, a "PharmaTrain Federation asbl" was formed in Belgium to be the long-term sustainable legal body for the organisation.
According to the decision taken during the 2017 General Assembly, the PharmaTrain Federation Switzerland has moved into closure when the final payment for the IMI-TRAIN project was received in December 2017. All the assets from the Swiss Federation moved into the Belgium Federation
The PharmaTrain Federation in Belgium has successully managed to become financially self-sustainable from its Assessments and Membership activities. It is now pursuing its mission to improve post-graduate competence development in Pharmaceutical Medicine/Medicines Development all around the world.
Board & Executives


President
A physician, specialized in General Medicine, Clinical Pharmacology and Pharmaceutical Medicine. Dr Klingmann held different senior medical, operational and managerial responsibilities in pharmaceutical industry, CROs and clinical trial sites, with focus on clinical trial design and management, ethical and regulatory aspects. Dr Klingmann is President of PharmaTrain Federation and Chairman of the Board of the European Forum for Good Clinical Practice (EFGCP). Her broad professional background as physician with experience in patient care, clinical development, site management and patient engagement enables Dr Klingmann to bridge the gaps between the interests and skills of all different stakeholders in medicines development with the aim to develop new patient-relevant treatments more efficiently. Dr Klingmann teaches on different clinical research and regulatory affairs topics in diploma and master courses at the University of Basel, Switzerland, the Université Libre de Bruxelles, Belgium, and the University of Bonn, Germany.

Vice-President
Professor Salek completed his undergraduate degree in Pharmacy at the University of Oklahoma, USA, in 1978. He later moved to Cardiff where he studied for his PhD, 1985–1989. Since completing his PhD, Professor Salek has held a number of academic posts on both sides of the Atlantic. Sam Salek is Professor of Pharmacoepidemiology in the School of Life and Medical Sciences, University of Hertfordshire, UK where he leads the Public Health & Patient Safety research group. He is also the Director of the Institute for Medicine Development, Cardiff, UK, and a visiting Professor at the State of Hessen, Germany. Professor Salek is the co-founded and past chair of the Patient Engagement Special Interest Group of the International Society of Quality of Research, co-chairs the European Hematology Association (EHA) Scientific Working Group for Quality of Life and Symptoms and chairs the EHA SWG 'Gaucher's Disease Task Force'. Professor Salek is a fellow of the Royal College of Physicians and the Royal Pharmaceutical Society of Great Britain.

Vice-President
Dr Birka Lehmann is Senior Expert for Drug Regulatory Affairs and lecturer at the University of Bonn since 1998.
Birka Lehmann studied Medicines at the Free University Berlin and trained at the Kinderklinik Norderney. Her working experience includes 9 years preclinical assessment in the division ‘Pharmacology and Toxicology’ of BfArM and she served as head of unit ‘Decentralised Procedure’ (1996-2002) and as deputy head of EU Division (2000-2002). She was member of and chaired the Mututal Recognition Facilitation Group and served as expert to the Committee for Human Medicinal Products (EMA).
From 2002 – 2006 she joined the European Commission, Directorate-General Enterprise and Industry as expert on secondment to in the unit ‘Pharmaceuticals’ responsible for inter alia Marketing Authorisation and implementation of Clinical Trials Directive. From September 2006 till October 2011 she was head of the division 3 Marketing Authorisation procedure at the BfArM compromising several indication areas including cardiovascular and pulmonary disease, antibiotics and dermatology.
She was head of Executive Department EU and International Affairs of the Federal Institute for Drugs and Medical Devices (BfArM) from October 2011 till end of 2015. She was member of the Paediatric Committee at the European Medicines Agency from 2007 till 2016.
Treasurer
Prof. Heinrich Klech is an internal medicine specialist by training from the University of Vienna. He is author of more than 200 publications in the field of lung diseases and served on the editorial board of many renowned medical journals in the field of Pulmonology and Internal medicine. He is a Fellow of the American College of Chest Physicians (FACP). Heinrich Klech is founder and managing director of the VSCR – Vienna School of Clinical Research, Public Health and Medical Education, an independent non-profit organization, specialized for post graduate education in clinical research, health outcome research and related sciences. Since 2012 Heinrich Klech serves as treasurer and member of the board of Pharmatrain. He was Pharmatrains mastermind behind the IMI Quality Initiative (supported by the European Commission and EFPIA) which firstly described European shared standards for training and education in biomedical science. As part of another European project, imi-train he led Pharmatrains activities for the development of the Specialist in Medicicine Development (SMD) which recently has been picked up also by Japan.

Secretary